Mass 30 g H2O2 x 100 100. Convert the following Molarities to Normalities.

Solved Be Sure To Answer All Parts Calculate The Molality Chegg Com
25 M HCl 25 N b.

. Answer Questions 1 2 and 3. Gravity of solution is 1g mL1. The Section II score weighting for each question is 20 percent.
Of moles of H2O Total no. For aqueous solutions at 20C 1 ppm corresponds to 1 μg per milliliter and 1 ppb corresponds to 1 ng per milliliter. As is evidenced in this example the concentration is reduced by a factor of ten in each step.
Many alloys ceramics and polymer blends are solid solutions. Assuming the density of the solution is 10 gcm3 calculate the molarity and molality of H2O2. Using S values from Appendix C calculate Δ S values for the following reactions.
The concentrations of very dilute solutions are often expressed in parts per million ppm which is grams of solute per 10 6 g of solution or in parts per billion ppb which is grams of solute per 10 9 g of solution. 05 M CaOH2 1 N 7. An analyst at Procter Gamble quality control department received a sample of toothpaste to A.
Molality moles of solute weight of solvent in g 1000. 14 M H2SO4 28 N c. Also determine the molarity of the solution specific.
Component in the solution. Do NOT write your answers on the green insert. Silver gold and copper form many different alloys with unique colors and appearances.
Mole fraction of H2O No. Each of three beakers contains 250 mL of a 0100 M solution of HCl NH. A salt water solution.
In many weak aqueous solutions the molarity and molality are similar because one kilogram of water the solvent occupies one liter of volume at room temperature and the small amount of solute has little effect on the volume of the solvent. Entropy is measurement of chaos in the thermodynamic system Here we are required to find the question_answer. A To determine the linear range in the graph.
If 4 g of NaOH dissolves in 36 g of H2O calculate the mole fraction of each. Within a certain range copper and zinc dissolve in each other and harden to give solid solutions called brass. 10 M NaOH 10 N d.
Serial dilutions are used to accurately create extremely diluted solutions as well as solutions for experiments that require a concentration curve with an exponential or. 1 M 01 M 001 M 0001 M and so on. A commonly purchased disinfectant is a 30 by mass solution of hydrogen peroxide H2O2 in water.
If the masses of the salt and of the water are known the. Alloys and other solid solutions are important in the world of materials chemistry. Be sure to write all your answers to the questions on the lined pages following each question in the booklet with the pink cover.
For example a ten-fold serial dilution could result in the following concentrations. Table salt readily dissolves in water to form a solution.

Solved Calculate The Molality Of Each Of The Following Chegg Com

Oneclass Calculate The Molality Of Each Of The Following Aqueous Solutions A 1 90 M Nacl Solution
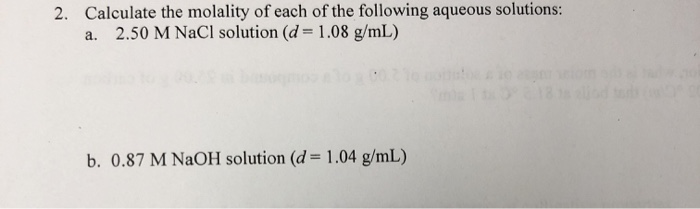
Solved 2 Calculate The Molality Of Each Of The Following Chegg Com
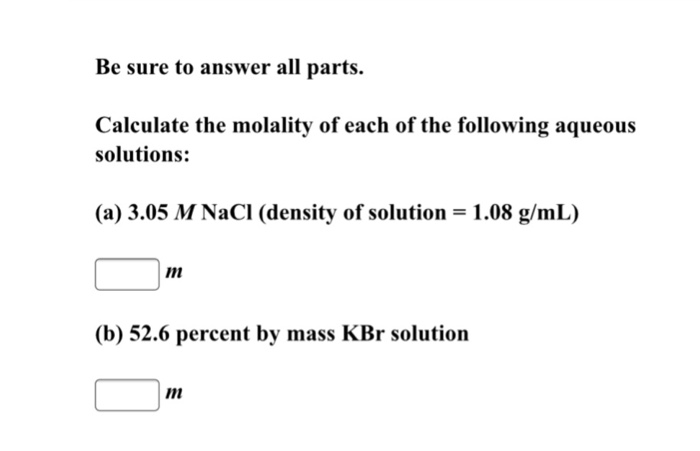
Solved Be Sure To Answer All Parts Calculate The Molality Of Chegg Com
0 Comments